BRAIN STEM AND POSTERIOR FOSSA
John R. Hesselink, MD, FACR and John F. Healy, MD, FACR
The posterior fossa houses the brainstem and cerebellum. The brainstem contains all the cranial nerve nuclei and many efferent and afferent fiber tracts that connect the brain with the rest of the body. The cerebellum is the major organ of coordination for all motor functions, as well as mental activities of the brain.
Many disease processes can occur in the posterior fossa. Tumors can arise from the brain tissue itself, the cranial nerves, the meninges, or the skull. All these structures are also susceptible to infection and inflammation. Vascular disease can lead to infarction or hemorrhage. Finally, the posterior fossa can be invaded by neoplasms and infections from the head and neck regions.
Magnetic resonance (MR) is ideally suited for imaging the posterior fossa. The high contrast
resolution and multiplanar capabilities of MR make possible accurate correlation of pathology with
the complex anatomy of this region. Gadolinium enhancement is often helpful to identify and
characterize lesions.
![]()
CRANIAL NERVES
The anatomy of the brainstem and cerebellum is very intricate. Some of the larger nuclei and fiber tracts are visible on MR images. Although others may not be visible, a general knowledge of the locations of major structures is essential to correlate the imaging findings with the clinical picture.
Anatomy
The cranial nerve nuclei are located in the tegmentum of the brainstem, just ventral to the
cerebral aqueduct and 4th ventricle. The 3rd nerves (oculomotor) pick up parasympathetic fibers
from the Edinger-Westfall nucleus and course ventrally through the substance of the midbrain to exit
in the interpeduncular cistern. The cisternal segments continue ventrally between the posterior
cerebral and superior cerebellar arteries and enter the cavernous sinuses. The 4th nerves (trochlear)
are the only cranial nerves to cross the midline. They course dorsally and cross behind the aqueduct,
exit the dorsal midbrain, and travel forward in the ambient cisterns to reach the cavernous sinuses.
Other major structures within the midbrain include the pyramidal (corticospinal and corticobulbar)
tracts within the cerebral peduncles, the substantia nigra, the red nuclei, the decussation of the
superior cerebellar peduncles, and the superior and inferior colliculi of the quadrigeminal plate.
![]()
The pons contains the nuclei for the 5th (trigeminal), 6th (abducens), 7th (facial), and the 8th
(acoustic) cranial nerves. The 5th nerve enters the mid-portion of the pons ventrolaterally. The
spinal tract and nucleus of the 5th nerve extends from the upper pons all the way down into the upper
spinal cord. The 6th exists ventrally at the pontomedullary junction. Both the 5th and 6th nerves
course through the cavernous sinus. The 7th nerve loops posteriorly around the 6th nerve nucleus
and indents the floor of the 4th ventricle (facial colliculus). The 7th and 8th nerves exist the inferior
pons inferiolaterally, traverse the cerebellopontine cistern and enter the internal auditory canal. The
anterior pons (basis pontis) contains a large number of transverse fibers from the middle cerebellar
peduncles and longitudinal, dispersed bundles of the pyramidal tracts.
![]()
The medulla contains the remaining cranial nerves. Nerves 9 (glossopharyngeal), 10 (vagus), and 11 (spinal accessory) exist laterally just posterior to the olivary nucleus and course toward the jugular foramen. The 12th cranial nerve (hypoglossal) exists the medulla ventral to the olive and courses ventrally to the hypoglossal canal. The medulla also contains the decussation of the pyramids (corticospinal tracts) ventrally and the inferior cerebellar peduncles posteriorly.
Two other important fiber tracts are the medial longitudinal fasciculus (MLF) and the medial lemniscus. The MLF, which connects the 3rd, 4th, and 6th cranial nerve nuclei, lies in a paramedian position just ventral to the aqueduct and 4th ventricle. The medial lemniscus, the major sensory tract, ascends through the brainstem just ventral to the MLF.
Pathology
Nerve sheath tumors
Tumors of schwann cell origin include schwannoma and neurofibroma. Schwannomas are more common and most arise from the 8th cranial nerve. Neurofibromas are usually associated with neurofibromatosis. Acoustic neuromas originate on the vestibular division of the eighth cranial nerve just within the internal auditory canal. Bilateral lesions are common with NF 2. They usually present in middle-aged adults with a sensorineural hearing loss, but other symptoms include headache, vertigo, tinnitus, unsteady gait, and facial weakness. Large tumors may fill the cerebellopontine angle cistern and compress adjacent brain structures, producing additional symptoms.
Most schwannomas are isointense to the brain on MR images, but some are distinctly
hyperintense with T2-weighted sequences. Occasionally, a schwannoma will be hyperintense on
T1-weighted images owing to foci of hemorrhage. They may be heterogeneous on T2-weighted
images as well, particularly the larger ones, due to necrosis, hemorrhagic components, and occasional
calcification. With small intracanalicular tumors, partial voluming effects may result in uneven signal
intensity.
![]()
Gadolinium causes approximately 50% shortening of the T1 relaxation time of schwannomas,
making them appear very bright on T1-weighted images. Those lesions that are heterogeneous on
plain scan will likely exhibit heterogeneous enhancement as well.
![]()
Meningioma
Meningiomas originate from the dura or arachnoid and occur in middle-aged adults. In the posterior fossa, most meningiomas are found in the cerebellopontine angle. Women are affected twice as often as men. Meningiomas are well-differentiated, benign, and encapsulated lesions that indent the brain as they enlarge. They grow slowly and may be present for many years before producing symptoms. The histologic picture shows cells of uniform size that tend to form whorls or psammoma bodies. They are hypervascular, receiving their blood supply predominantly from dural vessels.
Most meningiomas are isointense with cortex on T1- and T2-weighted images. A heterogeneous internal texture is found in all but the smallest meningiomas. The mottled pattern is likely due to a combination of flow void from vascularity, focal calcification, small cystic foci, and entrapped CSF spaces. Hemorrhage is not a common feature. An interface between the brain and the lesion is often present, representing a CSF cleft, a vascular rim, or a dural margin. MR has special advantages over CT in assessing venous sinus involvement and arterial encasement. Occasionally, a densely calcified meningioma is encountered that is distinctly hypointense on all pulse sequences.
Meningiomas show intense enhancement with gadolinium and are sharply circumscribed.
![]() They have a characteristic broad base of attachment against a dural surface. Contrast scans are
especially helpful for imaging the en plaque meningiomas that occur at the skull base.
They have a characteristic broad base of attachment against a dural surface. Contrast scans are
especially helpful for imaging the en plaque meningiomas that occur at the skull base.
Glomus Jugulare
These tumors arise from paraganglia along the auricular branch of the vagus nerve (nerve of Arnold) in the jugular fossa. They are benign but locally invasive lesions that slowly erode into adjacent areas of the skull base and posterior fossa. Most patients present with pulsatile tinnitus or neurological deficits secondary to involvement of cranial nerves 9, 10 and 11. Multiple tumors occur in about 10% of cases.
Paragangliomas have a unique MR appearance related to their extreme hypervascularity.
Serpiginous areas of signal void from blood flow are interspersed among areas of flow-related
enhancement and more isointense tumor cells. On T2-weighted images, they are of variable signal
intensity but usually hyperintense to the surrounding muscles. MR angiography is helpful in
determining involvement of the carotid artery and jugular vein. CT is better for showing subtle bone
erosions.
![]()
Epidermoid Tumors
Epidermoids are referred to as "pearly tumors" because of their glistening white appearance at surgery. They arise from epithelial cell rests in the basal cisterns. They are benign and grow slowly along the subarachnoid spaces and into the various crevices found at the base of the brain.
Intradural epidermoids are usually quite large with lobulated outer margins and an insinuating
pattern of growth. They have a heterogeneous texture and variable signal intensity on MR.
![]() Most
are slightly higher signal than CSF on both T1 and T2-weighted images. An occasional epidermoid
has a very short T1 and appears bright on T1-weighted images. The heterogeneous signal pattern
is likely related to varying concentrations of keratin, cholesterol, and water within the cyst, as well
as the proportion of cholesterol and keratin in crystalline form. Calcification is sometimes present.
Epidermoid tumors do not enhance with contrast. The cystic contents of epidermoids often exhibit
restricted diffusion on diffusion-weighted imaging.
Most
are slightly higher signal than CSF on both T1 and T2-weighted images. An occasional epidermoid
has a very short T1 and appears bright on T1-weighted images. The heterogeneous signal pattern
is likely related to varying concentrations of keratin, cholesterol, and water within the cyst, as well
as the proportion of cholesterol and keratin in crystalline form. Calcification is sometimes present.
Epidermoid tumors do not enhance with contrast. The cystic contents of epidermoids often exhibit
restricted diffusion on diffusion-weighted imaging.
Arachnoid Cyst
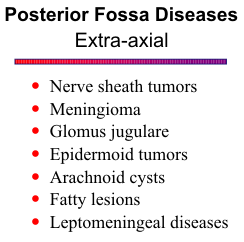
Arachnoid cysts are benign but slowly grow as they accumulate fluid, compressing normal
brain structures. Most are smoothly marginated and
homogeneous. They are not calcified and do not
enhance. The cyst fluid is usually isointense with CSF on
all pulse sequences. The cysts may appear higher signal
than CSF on intermediate T2-weighted images due to
dampening of the CSF pulsations that normally results in
signal loss in the ventricles and cisterns. This effect will
be less apparent with pulse sequences that incorporate
flow compensation techniques.
![]()
Fatty Lesions
Dermoid cysts and lipomas are unusual in the posterior fossa. Dermoids are primarily midline lesions, occurring near the torcula or the quadrigeminal plate. Lipomas are sometimes found in the cerebellopontine angle. Both are characterized by their fatty components on MR imaging.
Leptomeningeal Diseases
Neoplastic and inflammatory diseases can involve the leptomeninges. Leptomeningeal tumor
can develop from seeding of a malignant intracranial tumor or via a hematogenous route from an
extracranial site. Pediatric tumors that commonly seed the CSF include germinoma,
medulloblastoma, and pineoblastoma. In the adult, leptomeningeal carcinomatosis is usually
secondary to breast or lung carcinoma. Highly malignant gliomas can also seed the CSF.
![]()
Most bacterial meningitis is treated before changes become evident on imaging studies. The most common finding is a communicating hydrocephalus due to obstruction of the CSF pathways in the basal cisterns. The infectious agents that are more likely to show meningeal enhancement are tuberculosis and fungal infections, such as coccidioidomycosis. These organisms induce a vascular granulation tissue that readily enhances with gadolinium or iodinated contrast agents. A secondary vasculitis can also develop, leading to vessel occlusion and brain infarction.
Another inflammatory disease that produces a granulomatous reaction within the basal
meninges is sarcoidosis. CNS involvement occurs in about 5% of patients with sarcoidosis. The
pattern of meningeal involvement is usually more focal and patchy than with the infectious agents.
Vasculitis is less common, but the inflammation can extend along the Virchow-Robin spaces to enter
the brain substance.
![]()
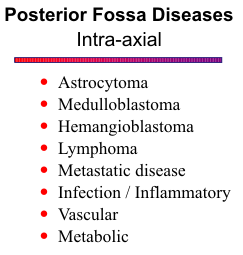
INTRAAXIAL TUMORS
Except for hemangioblastoma and metastatic disease, the majority of intra-axial posterior fossa tumors occur in children. Cerebellar astrocytoma accounts for 33% of these childhood tumors, medulloblastoma 26%, brain stem glioma 21%, ependymoma 14% and choroid plexus papilloma, only 2%.
Brain Stem Glioma
Most brain stem gliomas are relatively benign initially but frequently evolve to a higher grade. They usually present with a cranial nerve palsy, most often involving the 6th or 7th nerves. The pons is the common location, but they also occur in the medulla and midbrain. These tumors infiltrate the brain stem and induce surrounding vasogenic edema in the brain parenchyma. Since both the tumor and edema are hyperintense on T2-weighted images, tumor margins tend to be indistinct and poorly defined.
Brain stem gliomas are relatively homogeneous masses without much cystic change, necrosis, vascularity or calcification. About 50% of cases will show mild enhancement. As the gliomas grow, they enlarge the brain stem, producing effacement of the basal cisterns, anterior displacement of the basilar artery against the clivus, and compression and posterior bowing of the fourth ventricle. Hydrocephalus is often present. Exophytic growth is a well-known feature of these tumors.
Cerebellar Astrocytoma
Cerebellar astrocytoma is the most common CNS tumor in children. They tend to be lower grade than the supratentorial variety found in adults and are often quite large by time of presentation. The majority are hemispheric in location, a helpful but not absolute criterion to distinguish them from medulloblastoma.
More than 50% of cerebellar astrocytomas are cystic, and the cyst contents often have elevated protein, making them slightly higher signal than CSF but lower signal than brain on T1-weighted images. The solid components are hyperintense to brain on proton density-weighted images. Both solid tumor and cyst are bright on T2-weighted scans. Calcification is occasionally present. Peritumoral edema is not pronounced, and in general, their margins are defined better than in supratentorial gliomas. Cerebellar astrocytomas exhibit nodular or ringlike enhancement. Since these tumors are frequently large, mass effect is a prominent feature. Anterior and lateral displacement of the fourth ventricle is common. Upward herniation of the superior vermis and downward herniation of the cerebellar tonsils can also occur.
Medulloblastoma (and PNET)
The majority of medulloblastomas occur in children between four and eight years old, and
males outnumber females three to one. Primitive neuro-ectodermal tumors (PNET) may present at
birth or early infancy. Medulloblastomas and PNETS arise from remnants of primitive
neuro-ectoderm in the roof of the fourth ventricle. These tumors are very malignant and exhibit an
aggressive biologic behavior, commonly invading the adjacent brain stem and leptomeninges.
Widespread dissemination through the ventricular system and distant seeding to other areas of the
neuraxis occurs in as high as 30%.
![]()
Medulloblastomas are primarily midline vermian lesions, but hemispheric locations are also possible.
Since they arise close to the fourth ventricle, growth predominantly into the ventricle may make them
simulate an intraventricular mass. Necrosis, hemorrhage and cavitation are common features, giving
these tumors a heterogeneous appearance on MR, but not to the same degree as seen with
ependymomas. Calcification is rare in medulloblastomas.
![]() ,
,
![]() They are hypervascular lesions and show
moderate contrast enhancement.
They are hypervascular lesions and show
moderate contrast enhancement.
Ependymoma
About 70% of ependymomas are found in the fourth ventricle. The atria of the lateral
ventricles are another common site. Males are affected twice as often as females. They originate from
the ependyma of the ventricles but may grow either into the ventricle or into the brain substance.
Ependymomas are slow-growing, but malignant, tumors and grow by expansion and infiltration.
Ventricular and subarachnoid seeding are not infrequent.
![]()
Most ependymomas arise in the floor of the fourth ventricle. They have a propensity to extend
through the foramina of Luschka and Magendie into the basal cisterns. They tend to be well defined,
particularly if they are marginated by CSF within a ventricle or cistern. Calcification is present in
50%, cysts and necrotic areas are common, and most are moderately vascular. These properties
account for their heterogeneous internal texture on both plain and contrast scans.
![]()
Choroid Plexus Papilloma
Choroid plexus papillomas are rare tumors that arise from cells of the choroid plexus. Their histologic picture is strikingly similar to normal choroid plexus. These lesions are benign and expand within a ventricle, eventually leading to obstruction of CSF pathways. Moreover, they may be associated with increased CSF production, another factor contributing to hydrocephalus.
Choroid plexus papillomas usually have well-defined margins, with parts of the tumor outlined by
CSF. On T2-weighted scans they are only mildly hyperintense because their T2 relaxation times are
not as prolonged as in parenchymal tumors. They are relatively homogeneous, but hypervascularity
can result in areas of flow void or flow enhancement. Choroid plexus papillomas demonstrate intense
homogeneous contrast enhancement.
![]()
Hemangioblastoma
Hemangioblastoma is a benign tumor of middle age. In fact, it is the most common primary intra-axial tumor of the posterior fossa in adults. About 20% are associated with Hippel-Lindau disease, and hereditary factors have been implicated in another 20%. The cerebellum and vermis are the common sites, but hemangioblastomas can also be found in the medulla and spinal cord. Multiplicity is a well-known feature but is present in only about 10% of cases. Histologic examination reveals a meshwork of capillaries and small vessels.
The classic MR appearance of hemangioblastoma is a cystic mass with a brightly enhancing nodule.
About 60% are cystic, so solid lesions are not uncommon. Calcification is rare. Hemangioblastomas
are sharply marginated and induce minimal surrounding parenchymal reaction. The tumor nodules are
hypervascular and the vascular pedicle often produces a characteristic flow void on MR.
![]()
Lymphoma
Primary malignant lymphoma is a non-Hodgkin's lymphoma that occurs in the brain in the absence of systemic involvement. Most occur in the setting of immune suppression related to HIV infection, chemotherapy, or immunosuppressive therapy for organ transplantation. These tumors are highly cellular and grow rapidly. Cerebral lymphomas are very radiosensitive and respond dramatically to steroid therapy.
Lymphomas typically appear as homogeneous, slightly high signal to isointense masses on
T2-weighted images. The observed mild T2 prolongation is probably related to dense cell-packing
within these tumors, leaving relatively little interstitial space for accumulation of water. Multiple
lesions are present in as many as 50%. Despite their rapid growth, central necrosis is uncommon.
They are associated with only a mild or moderate amount of peritumoral edema. By time of
presentation they can be quite large and yet produce relatively little mass effect, a feature that sets
lymphoma apart from glioblastoma and metastases. Intratumoral cysts and hemorrhage are unusual.
Most lymphomas show bright homogeneous contrast enhancement.
![]()
The pattern is modified somewhat in AIDS patients. Multiplicity seems to be more common.
Moreover, lymphomas exhibit more aggressive behavior and readily outgrow their blood supply. As
a result, central necrosis and ring enhancement are often seen in lymphomatous masses in AIDS
patients.
![]()
Metastatic disease
Metastases to the brain occur by hematogenous spread, and multiple lesions are found in 70% of cases. The most common primaries are lung, breast, and melanoma, in that order of frequency. Other potential sources include the gastrointestinal tract, kidney, and thyroid. Metastases from other locations are uncommon. Clinical symptoms are nonspecific and no different from primary brain tumors. If a parenchymal lesion breaks through the cortex, tumor can extend and seed along the leptomeninges.
Metastatic lesions can be found anywhere in the brain but a favorite site is near the brain surface at the corticomedullary junction of both the cerebrum and cerebellum. They are hyperintense on plain T2-weighted images. Areas of necrosis are prevalent in the larger lesions, accounting for their heterogeneous internal texture. Peritumoral edema is a prominent feature, but multiplicity is the most helpful sign to suggest metastatic disease as the likely diagnosis. Hemorrhage is present in 3 to 14% of brain metastases, mainly in melanoma, choriocarcinoma, renal cell carcinoma, bronchogenic carcinoma, and thyroid carcinoma. The presence of nonhemorrhagic tissue and pronounced surrounding vasogenic edema are clues to the underlying neoplasm.
Gadolinium enhanced MR results in improved delineation of metastatic disease compared with
nonenhanced scans. Moderate to marked enhancement is the rule, nodular for the smaller lesions and
ringlike with central nonenhancing areas for the larger ones.
![]() Correlative studies have shown MR
to be more sensitive than CT for detecting metastases, particularly lesions near the base of the brain
and in the posterior fossa.
Correlative studies have shown MR
to be more sensitive than CT for detecting metastases, particularly lesions near the base of the brain
and in the posterior fossa.
REFERENCES