CEREBRAL GLIOMAS

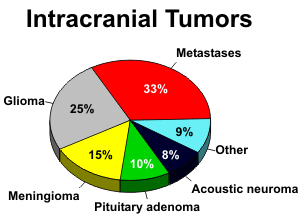
Gliomas are malignant tumors of the glial cells of the brain and account for 30-40% of all primary intracranial tumors. They occur predominantly in the cerebral hemispheres, but the brain stem and cerebellum are frequent locations in children, and they are also found in the spinal cord. The peak incidence is during middle adult life, when patients present with seizures or symptoms related to the location of the gliomas and the brain structures involved.
Astrocytomas are graded according to their histologic appearance. Grade 1 astrocytomas have well-differentiated astrocytes and well-defined margins. The clinical course often proceeds over many years and complete cures are possible. The pilocytic variant is a low-grade tumor with a distinct capsule that is commonly found in children. The giant cell astrocytoma is a specialized tumor that develops from pre-existing hamartomas in patients with tuberous sclerosis. Grade 2 astrocytomas are well-differentiated but diffusely infiltrating tumors. The fibrillary type is most common, and although initially benign, they may evolve into a higher grade tumor over time. This changing character of gliomas makes histological classification difficult from sample biopsies, because different parts of the tumor often exhibit varying degrees of malignancy. The higher grade astrocytomas are very cellular and pleomorphic. Anaplastic astrocytomas (Grade 3) are very aggressive tumors, readily infiltrate adjacent brain structures, and have a uniformly poor prognosis. Glioblastoma multiforme (Grade 4) has the added histologic features of endothelial proliferation and necrosis. Multicentric foci of tumor may be seen in 4 to 6% of glioblastomas. Gliomatosis cerebri is an unusual condition with diffuse contiguous involvement of multiple lobes of the brain.
Oligodendrogliomas are the most benign of the gliomas. Calcification is common, and they
occur predominantly in the frontal lobes.
![]() The mixed neuronal and glial tumors are found mostly
in children and young adults. They are slow-growing and are found predominantly in the temporal
lobes and around the third ventricle. Intratumoral cysts and calcification are common.
The mixed neuronal and glial tumors are found mostly
in children and young adults. They are slow-growing and are found predominantly in the temporal
lobes and around the third ventricle. Intratumoral cysts and calcification are common.
![]()
The common signal characteristics of intra-axial tumors include high signal intensity on T2-weighted images and low signal on T1-weighted images, unless fat or hemorrhage is present. Fat and subacute hemorrhage (methemoglobin) exhibit high signal on T1-weighted images, and acute hemorrhage (deoxyhemoglobin) and chronic hemorrhage (hemosiderin/ferritin) show low signal intensity on T2-weighted scans. Gliomas have poorly defined margins on plain MR. They infiltrate along white matter fiber tracts, and the deeper lesions have a propensity to extend across the corpus callosum into the opposite hemisphere. They are often quite large by the time of clinical presentation.
The higher grade gliomas, particularly glioblastomas, appear heterogeneous due to central
necrosis with cellular debris, fluid, and hemorrhage. Peritumoral edema and mass effect are common
features. Following injection of gadolinium, T1-weighted images show irregular ring enhancement,
with nodularity and nonenhancing necrotic foci.
![]() As mentioned above, gliomas are infiltrative lesions,
and microscopic fingers of tumor usually extend beyond the margin of enhancement. Enhanced scans
are particularly helpful to outline subependymal spread of tumor along a ventricular surface, as well
as leptomeningeal involvement.
As mentioned above, gliomas are infiltrative lesions,
and microscopic fingers of tumor usually extend beyond the margin of enhancement. Enhanced scans
are particularly helpful to outline subependymal spread of tumor along a ventricular surface, as well
as leptomeningeal involvement.
![]() Although highly malignant, anaplastic astrocytomas may or may not
exhibit breakdown of the blood-brain barrier. In general, the presence or lack of enhancement alone
is not helpful in grading astrocytomas.
Although highly malignant, anaplastic astrocytomas may or may not
exhibit breakdown of the blood-brain barrier. In general, the presence or lack of enhancement alone
is not helpful in grading astrocytomas.
The lower grade astrocytomas tend to be more homogeneous without central necrosis. Large cystic components may be present. The cysts have smooth walls, and the fluid is of uniform signal, to distinguish them from necrosis. Enhancement is variable, depending on the integrity of the blood-brain barrier.
Perfusion imaging has shown promise as a technique for determining the grade of intracranial
mass lesions. Perfusion imaging relies on a first-pass susceptibility-related signal loss on
T2*-weighted images, from which relative cerebral blood flow and volume can be calculated. Several
studies have shown a correlation between relative cerebral blood volume and tumor grade, likely due
to the relationship of blood volume to vascular proliferation in high-grade gliomas.
![]()
MR Spectroscopy
MR spectroscopy provides a measure of brain chemistry and can help characterize tumors and and grade the degree of malignancy. As a general rule, as malignancy increases, NAA and creatine decrease, and choline, lactate, and lipids increase. NAA decreases as tumor growth displaces or destroys neurons. Very malignant tumors have high metabolic activity and deplete the energy stores, resulting in reduced creatine. Very hypercellular tumors with rapid growth elevate choline. Lipids are found in necrotic portions of tumors, and lactate appears when tumors outgrow their blood supply and start utilizing anaerobic glycolysis. To get an accurate assessment of the tumor chemistry, the spectroscopic voxel should be placed over an enhancing region of the tumor, avoiding areas of necrosis, hemorrhage, calcification, or cysts.
Multi-voxel spectroscopy is best to detect infiltration of malignant cells beyond the enhancing
margins of tumors. Particularly in the case of cerebral glioma, elevated choline levels are frequently
detected in edematous regions of the brain outside the enhancing mass.
![]() Finally, MRS can direct the
surgeon to the most metabolically active part of the tumor for biopsy to obtain accurate grading of
the malignancy.
Finally, MRS can direct the
surgeon to the most metabolically active part of the tumor for biopsy to obtain accurate grading of
the malignancy.
A common clinical problem is distinguishing tumor recurrence from radiation effects several months following surgery and radiation therapy. Elevated choline is a marker for recurrent tumor. Radiation change generally exhibits low NAA, creatine, and choline on spectroscopy. If radiation necrosis is present, the spectrum may reveal elevated lipids and lactate.
MRS cannot always distinguish primary and secondary
tumors of the brain from one another. As mentioned above, one
key feature of gliomas is elevated choline beyond the margin of
enhancement due to infiltration of tumor into the adjacent brain
tissue. Most non-glial tumors have little or no NAA. Elevated
alanine at 1.48 ppm is a signature of meningiomas. They also
have no NAA, very low creatine, and elevated glutamates.
![]()
{To return to cases, use the "Back " button on the Toolbar}