IMAGING OF STROKE AND CEREBRAL ISCHEMIA
John R. Hesselink, MD, FACR
The importance of occlusive cerebral vascular disease is confirmed by two facts: 1) stroke is the third commonest cause of death in the United States; and 2) one-half of neurology inpatients have stroke related problems. These patients present with either asymptomatic bruits, transient ischemic attacks (TIA), or stroke. A TIA is a transient neurologic deficit lasting from a few seconds to a few hours. Another terminology that is sometimes used is reversible ischemic neurologic deficit (RIND), which refers to a neurological deficit that lasts longer than 24 hours, but results in complete recovery. These patients are often included in the larger category of stroke. The neurological deficit may consist of visual disturbances (transient monocular blindness or amaurosis fujax), weakness or numbness in an extremity or aphasia. Strokes in a carotid territory usually result in a contralateral hemiparesis and, if in the dominant hemisphere, an aphasia. Vertebral-basilar ischemia results in dizziness, diplopia, dysarthria and weakness or numbness that does not lateralize well.
CAUSES OF STROKE
The five major causes of cerebral infarction are vascular thrombosis, cerebral embolism, hypotension, hypertensive hemorrhage, and anoxia/hypoxia. Thrombotic strokes may occur abruptly but the clinical picture often shows gradual worsening over the first few hours. Primary causes of arterial thrombosis include atherosclerosis, hypercoagulable states, arteritis, and dissection. Secondary compromise of vascular structures can result from traumatic injury, intracranial mass effect, neoplastic encasement, meningeal processes, and vasospasm.
Embolic strokes characteristically have a very abrupt onset. After a number of hours, there may be sudden improvement in symptoms as the embolus lyses and travels more distally. The source of the embolus is usually either the heart (patients with atrial fibrillation or previous myocardial infarction) or ulcerated plaques at the carotid bifurcation in the neck.
Hypotension can be cardiac in origin or result from blood volume loss or septic shock. Hypertension can cause a primary intracerebral hemorrhage, or the elevated arterial pressure can overwhelm the brain's autoregulatory mechanism, resulting in breakthrough of the blood-brain barrier and brain edema. The latter phenomenon of hypertensive encephalopathy is a potential complication of eclampsia, but is usually transient and reversible. Anoxia/hypoxia events are usually related to respiratory compromise from severe lung disease, perinatal problems, near drowning, high altitude, carbon monoxide inhalation, or CNS mediated effects.
PHYSIOLOGY, METABOLISM, AND PATHOLOGY
The brain requires glucose and oxygen to maintain neuronal metabolism and function. Hypoxia refers to inadequate delivery of oxygen to the brain, and ischemia results from insufficient cerebral blood flow (CBF). Normal CBF is 50-55 ml/100gm/min. If the CBF drops below 18, electrical activity ceases. If the CBF dips below 10, neuronal metabolism stops.
The consequences of cerebral ischemia depend on the degree and duration of reduced CBF. Neurons can tolerate ischemia for 30-60 minutes. Perfusion must be reestablished before 3-6 hours of ischemia have elapsed or before the CBF drops to 10. The range of CBF between 10 and 18 has been called the "ischemic penumbra" because the neuronal damage is mild and reversible if flow is restored within a few hours. Clinically, a window of opportunity is available to intervene therapeutic and to prevent the ischemic brain tissue from going on to infarction.
If flow is not reestablished to the ischemic area, a series of metabolic processes ensue. The neurons become depleted of ATP and switch over to anaerobic glycolysis, a much less efficient pathway. Lactate accumulates and the intracellular pH decreases. Without an adequate supply of ATP, the membrane ion pump fails. There is an influx of sodium, water, and calcium into the cell. The excess calcium is detrimental to cell function and contributes to membrane lysis. Cessation of mitochondrial function signals neuronal death. The astrocytes and oligodendroglia are slightly more resistant to ischemia, but their demise follows shortly if blood flow is not restored.
Pathologic changes within the neuropil follow the metabolic abnormalities. One of the first effects
is cytotoxic edema that results from failure of the Na/K ion pump. Early on, this stage is still
reversible. Prolonged ischemia leads to cell death and coagulation necrosis. After 3-6 hours of
ischemia, irreversible damage occurs to the capillary endothelium. The blood-brain barrier becomes
dysfunctional and serum proteins and water leak into the interstitial space. Some reperfusion is
required to produce vasogenic edema. In fact, vasogenic edema is maximal when residual CBF is
between 5 and 10. There is also an influx of macrophages to clean up the dead tissues. Capillary
proliferation begins near the end of the first week. The end result of cerebral infarction is an area of
encephalomalacia with some surrounding gliosis. The amount of gliosis depends on the number of
surviving astrocytes.
![]()
CT AND MR IMAGING
Acute Infarcts
CT and MR scans in patients with asymptomatic bruits or TIA's are usually negative, unless they disclose abnormalities related to previous events. In patients with stroke, the earliest sign may be abnormal vascular density/signal. Acute thrombus or embolus is hyperdense on CT. Acute clot may be difficult to detect on MR, but the occluded artery should be apparent by the absence of a normal flow void. The absent flow void is easiest to see in the larger arteries at the base of the brain on T2-weighted images. It is not possible to conclusively distinguish a complete occlusion from a critical stenosis with markedly reduced flow. Subacute clot is hyperintense and is easiest to visualize in the basilar and middle cerebral arteries on T1-weighted images. One must be careful not to mistake in-flow enhancement with intraluminal clot. This phenomenon is most often observed in the end slices of a multislice set in arteries with slow flow entering the imaging volume.
Another valuable sign of acute stroke is arterial enhancement. With slow arterial flow, the spin-echo is able to capture the intravascular signal, and the T1 shortening effect of the gadolinium renders
the arteries hyperintense on T1-weighted images. Arterial enhancement is more apparent in the
smaller distal branches. It will be present in up to 45% of patients during the first week.
![]()
The first parenchymal changes observed on CT and MR reflect the cytotoxic edema affecting
primarily the gray matter. It is important to remember that the CT scan may be negative for the first
24-36 hours. Massive infarctions may be visible as early as 6 hours. The MR scan is usually positive
within three to four hours following a stroke. One of the earlier signs on CT is loss of the normal
gray-white contrast as the edematous cortex becomes isodense to the underlying white matter. A
similar phenomenon is not observed on MR because the increased water in the gray matter renders
the cortex higher signal on T2-weighted images and lower signal on T1-weighted images, thereby
increasing gray-white contrast. It is often easier to appreciate the increased cortical signal on proton
density-weighted images. The cortical swelling is more apparent on T1-weighted scans. Cortical
edema produces effacement of the sulci on both CT and MR.
![]()
After 6-8 hours the accompanying vasogenic edema highlights the areas of brain infarction. These fluid shifts are more profound and are responsible for effacement of the ventricles and midline shifts. The mass effect increases over the first few days and becomes maximal at about five days.
Incomplete ischemia has received special attention in the neuroradiologic literature. It results
from transient interruption of the blood supply from iatrogenic causes or from an embolus that breaks
up and rapidly restores perfusion to the area. The distinctive features of incomplete ischemia are early
intense parenchymal enhancement and little or no arterial enhancement. If arterial enhancement is
present, it disappears within 24-48 hours. The parenchymal enhancement results from a combination
of reperfusion and vasomotor deregulation or vasodilatation. This "luxury of perfusion" last about
24 hours. There may be some mild brain edema during the first 24-48 hours, but the parenchymal
signal changes are minimal or absent. Incomplete ischemia is associated with a good prognosis.
![]()
Subacute and Chronic Infarcts
The subacute stage begins during the second week with capillary proliferation in the area of infarcted brain tissue. This neovascularity is devoid of any blood-brain barrier and intravascular contrast freely diffuses into the interstitial spaces. The serpiginous character of the gyral enhancement is quite distinctive of cerebral infarction. A focal cerebritis or encephalitis can mimic this pattern, but usually the clinical picture sets apart these entities. Following contrast infusion, infarcts will typically enhanced between 2 and 8 weeks, but the enhancement can persist for up to three months.
As an infarct evolves, it becomes progressively lower in density on CT (higher in signal on T2-weighted images) and more well defined over the next few weeks, eventually approaching the density of CSF. As the mass effect resolves and the infarcted tissue is resorbed, the adjacent sulci and ventricle will enlarge. The end result is a chronic infarct with focal areas of cystic encephalomalacia and some surrounding parenchymal change due to gliosis.
Vascular Patterns
Since most infarcts result from occlusion of vessels, the CT or MR pattern of abnormality should follow one of the major vascular territories, such as the anterior cerebral, middle cerebral or posterior cerebral arteries. Infarcts can usually be distinguished from inflammatory and neoplastic disease because unlike the white matter pattern of edema found with tumors and abscesses, infarcts involve the cortex as well and, therefore, the abnormal density or signal intensity should extend peripherally to involve the cortex. As mentioned above, the enhancement pattern of infarcts is also fairly characteristic, having a gyral pattern of enhancement along the cortex. If a stroke is due to systemic hypotension or hypoxia, the area of infarction is commonly found in watershed areas between the major vascular territories.
Lacunar infarction results from occlusion of the small penetrating arteries at the base of the brain,
including the lenticulostriate and thalamoperforating arteries. They are smaller infarcts (less than 1
cm) and are found in the basal ganglia, thalamus and brainstem. MR is far more sensitive than CT
for detecting small lacunar infarcts, particularly in the brainstem where CT scans are often degraded
by artifacts from the bone at the skull base.
![]()
Hemorrhagic Stroke
The four major causes of hemorrhagic stroke are hypertension, hemorrhagic infarction, hypocoagulable state, and amyloid angiopathy. The criteria for hypertensive hemorrhage include a hypertensive patient, 60 years of age or older, and a basal ganglia or thalamic location of the hemorrhage. A CT scan is the procedure of choice for evaluating these patients. Arteriography is necessary only if one of these criteria is missing. Hypertensive hemorrhages are often large and devastating. Since they are deep hemorrhages and near ventricular surfaces, ventricular rupture is common. One-half of hypertensive hemorrhages occur in the putamen; the thalamus in 25%; pons and brainstem, 10%; cerebellum, 10%, and cerebral hemispheres, 5%.
In stroke patients, despite the fact that the CT is often negative for the first 24-48 hours, it is
often obtained on the day of admission to exclude an intracerebral hemorrhage before the patient is
placed on anticoagulant therapy. Hemorrhage into an infarct can occur during the first week, usually
between the third and fifth days. Hemorrhagic infarction is a hallmark of embolic infarction. This
occurs after the embolus breaks up, resulting in reperfusion of the infarcted area. As mentioned
above, hemorrhage is also common with venous infarction.
![]()
Amyloid angiopathy occurs in the elderly, generally over 70 years of age, and is associated with Alzheimer's disease. It results from deposition of eosinophilic material in the media and adventitia of small arteries and arterioles of the cortex and leptomeninges. Fibrinoid degeneration and microaneurysmal dilatation lead to vessel rupture and hemorrhage. The typical imaging pattern is multiple, usually relatively small, cortical hemorrhages that spare the white matter and cerebellum.
Venous and Dural Sinus Thrombus
Occlusion of the venous sinuses results in cerebral venous engorgement, brain swelling, and increased intracranial pressure. If the thrombosis extends retrograde and involves the cortical veins, secondary cerebral infarction can occur.
Acute thrombus is hyperdense on CT and may be
detected within one of the major sinuses or cortical veins.
The other classic sign is the "empty delta" sign due to
nonfilling of the superior sagittal sinus on a contrast scan.
Nonetheless, MR is far superior for diagnosing
abnormalities of the cerebral veins and sinuses. Normally,
the dural sinuses have sufficient flow to exhibit a flow
void. If that flow void is missing or if the sinuses are
hyperintense, thrombosis should be suspected. One must
be careful to exclude the possibility of any in-flow
enhancement effect. The diagnosis must be confirmed
with gradient-echo techniques or MR angiography. Phase-contrast MRA is the preferred technique because it is not adversely affected by intraluminal clot.
![]()
Associated parenchymal infarcts are found in the areas of venous abnormalities, and the infarcts are often hemorrhagic because arterial perfusion is maintained to the damaged tissue. In cases of superior sagittal sinus thrombosis, the infarcts are typically bilateral and in a parasagittal location.
DIFFUSION & PERFUSION IMAGING
Conventional CT and MR imaging are not sufficiently sensitive to evaluate acute stroke. CT is perfectly adequate to detect intracranial hemorrhage, but in the case of nonhemorrhagic stroke, the CT scan may be negative for the first 24 to 36 hours. FLAIR and T2-weighted images can detect acute stroke by 6 to 12 hours, but most new stroke therapies focus on the first 3 hours after onset. The ultimate goal for imaging is to define the area of brain infarction and perfusion deficit, and to identify any ischemic tissue that can be salvaged by medical or surgical therapy. Diffusion-weighted imaging can detect acute brain infarction within 1 to 2 hours. Perfusion imaging is positive immediately following an acute stroke.
Diffusion-Weighted Imaging
Diffusion imaging makes use of the variability of “Brownian motion” of water molecules in brain tissue. Brownian motion refers to the random movement of molecules. Water molecules are in constant motion, and the rate of movement or diffusion depends on the kinetic energy of the molecules and is temperature dependent. In biological tissues, diffusion is not truly random because tissue has structure. Cell membranes, vascular structures, and axon cylinders, for example, limit or restrict the amount of diffusion. Also, chemical interactions of water and macromolecules affect diffusion properties. Therefore, in the brain, water diffusion is referred to as “apparent diffusion.”
To obtain diffusion-weighted images, a pair of strong gradient pulses are added to the pulse sequence. The first pulse dephases the spins, and the second pulse rephases the spins if no net movement occurs. If net movement of spins occurs between the gradient pulses, signal attenuation occurs. The degree of attenuation depends on the magnitude of molecular translation and diffusion weighting. The amount of diffusion weighting is determined by the strength of the diffusion gradients, the duration of the gradients, and the time between the gradient pulses.
Diffusion imaging is performed optimally on a high-field (1.5 T) echo-planar system, but it can be accomplished with a turboSTEAM sequence on systems with conventional gradients.
The diffusion data can be presented as signal intensity or as an image map of the apparent diffusion coefficient (ADC). Calculation of the ADC requires 2 or more acquisitions with different diffusion weightings. A low ADC corresponds to high signal intensity (restricted diffusion), and a high ADC to low signal intensity on diffusion-weighted images.
In the setting of acute cerebral ischemia, if the cerebral blood flow is lowered to 10 ml/100gm/min, the cell membrane ion pump fails and excess sodium enters the cell, which is followed by a net
movement of water from the extracellular to intracellular compartment and cytotoxic edema.
Diffusion of the intracellular water molecules is restricted by the cell membranes. The restricted
diffusion results in a decreased ADC and increased signal intensity on diffusion-weighted images.
Severe ischemia can lower the ADC by as much as 56% of normal tissue at 6 hours.
![]()
In patients who present with symptoms of cerebral ischemia, diffusion-weighted images are very
helpful to identify any area of acute ischemia and to separate the acute infarction from old strokes and
other chronic changes in the brain. Only the acute infarcts appear hyperintense on the diffusion
images. Subacute and chronic infarcts,
vasogenic edema, the punctate and confluent
changes of deep white matter ischemia, and
dilated VR spaces are not bright.
![]() Bacterial
abscesses may exhibit restricted diffusion due
to thick cellular debri within the central
cavity. Other diseases of the brain, such as
non-bacterial infections, neoplasia,
contusions, and demyelinating diseases, are
not associated with cytotoxic edema, and
therefore as a rule, they are not hyperintense
on the diffusion images. One exception is
epidermoid tumors, which have restricted
diffusion due to the waxy consistency of their
contents. Also, the central portions of some
primary and secondary brain tumors may
exhibit restriction diffusion as they outgrow
their blood supply and become ischemic.
Occasionally, an acute MS plaque may be mildly hyperintense with diffusion weighting.
Bacterial
abscesses may exhibit restricted diffusion due
to thick cellular debri within the central
cavity. Other diseases of the brain, such as
non-bacterial infections, neoplasia,
contusions, and demyelinating diseases, are
not associated with cytotoxic edema, and
therefore as a rule, they are not hyperintense
on the diffusion images. One exception is
epidermoid tumors, which have restricted
diffusion due to the waxy consistency of their
contents. Also, the central portions of some
primary and secondary brain tumors may
exhibit restriction diffusion as they outgrow
their blood supply and become ischemic.
Occasionally, an acute MS plaque may be mildly hyperintense with diffusion weighting.
Lesions with prolonged T2 relaxation times are commonly mildly hyperintense on diffusion-weighted images. This phenomenon of “T2 shine-through” can easily be distinguished from true restricted diffusion on the ADC map. Only true restricted diffusion is low signal on the ADC map.
Perfusion Imaging
Measurements of brain perfusion include vascular transit time, cerebral blood volume, and cerebral blood flow. The ultimate goal is absolute cerebral blood flow, but it requires serial analysis of arterial input. Most methods measure relative blood flow and compare the two hemispheres or the individual lobes of the brain for regional differences. Both PET and Xenon CT are good techniques, but neither is widely used. PET requires access to a cyclotron and injection of a radioisotope. Xenon CT can be done on a conventional scanner with some additional hardware and software, but use of the xenon gas is cumbersome and the gas must be disposed of properly.
Vascular transit time and cerebral blood volume are less elegant than cerebral blood flow, but they are useful measurements and can be obtained easily on conventional CT and MR systems with a single bolus injection of contrast material. For CT 40 ml of contrast (300-370 ml I/ml) is injected at a rate of 5-8 ml/sec. Depending on the scanner configuration and number of detector rows, 2-4 sections, 5-10 mm thick are obtained. One image set is acquired per second for 40 seconds. As the bolus of contrast material passes through the cerebral circulation, a transient increase in attenuation or density occurs. A time-density curve is generated for each voxel in each CT slice (see diagram). From the curves, several perfusion image maps can be produced. The time-to-peak (TTP) is the time from the start of the scan until maximum attenuation occurs. The mean transit time (MTT) is the time it takes the contrast bolus to pass from the arterial to the venous side of the cerebral circulation. Assuming a symmetrical attenuation curve, the MTT is measured from the first detection of contrast to the maximum attenuation. The entire area under the curve is a measure of relative cerebral blood volume (rCBV). Finally, a measure of relative cerebral blood flow (rCBF) is calculated by dividing the rCBV by the MTT.
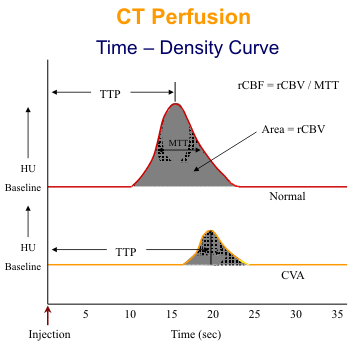
For MR perfusion, 20 ml of gadolinium is injected at 4ml/sec. An echoplanar sequence is used to acquire 10- 15 slices every 2-3 seconds for 40 second acquisition time. As the paramagnetic gadolinium bolus passes through the cerebral circulation, signal attention occurs due to T2* effects. The time- intensity curves for EPI perfusion are similar to the time-density curves for CT except that the curves are inverted.
TTP and MTT are very sensitive to hemodynamic changes in the cerebral circulation. Mild increases of these parameters with normal rCBF and rCBV likely indicate that no brain tissue is at immediate risk of infarction. When progresses arterial obstruction, the rCBF decreases followed by the rCBV. The rCBV initially remains normal or even increases due to autoregulation and dilatation of the capillary bed. If both rCBF and rCBV are reduced, tissue must be assumed. If both parameters are markedly reduced, irreversible ischemia has likely occurred.
Another MR perfusion method is called EPI signal targeting with alternating radiofrequency
(EPISTAR). In-flowing blood is tagged with a 180 degree pulse during alternate acquisitions through
the imaging plane of interest. The tagged and untagged images are subtracted to obtain the blood
volume map.
![]()
Diffusion/Perfusion Mismatch
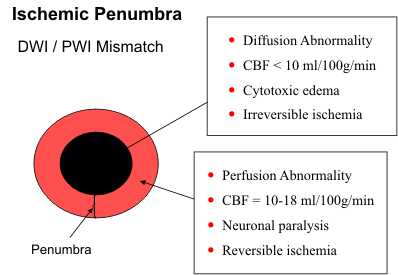
The difference between the diffusion and perfusion abnormalities provide a measure of the ischemic
penumbra or the brain tissue at risk for infarction (see diagram). Restricted diffusion generally
indicates unsalvageable brain tissue
that is destined for infarction. The
perfusion abnormality encompasses
all regions with reduced cerebral
blood flow. If the diffusion
abnormality matches the area of
decreased perfusion, no salvageable
ischemic brain tissue is present. If
the perfusion abnormality is larger
than the area of restricted diffusion,
the difference identifies the region of
reversible ischemia that can be saved
if blood flow is re-established
promptly.
![]()
VASCULAR IMAGING
The approach to imaging the cerebral circulation varies from one institution to another. It depends
on regional philosophies of therapy in occlusive vascular disease and on the relative strengths of the
different imaging procedures at the institution. In any case, one needs to have a rational approach
and I will present you with one such approach. If a reliable noninvasive laboratory is available,
preferably with duplex Doppler capability, asymptomatic bruits should be evaluated in the noninvasive
laboratory. If these results are positive or equivocal, then one should proceed to an angiographic
study, either MR or CT angiography. As the technology advances, MRA and CTA are assuming more
prominent roles in evaluating occlusive vascular disease. If a patient presents with a stroke, a similar
algorithm can be followed. The controversy arises in the patients who present with TIA's. If a
patient has had a single TIA, I think it is reasonable to follow these patients in the noninvasive
laboratory, but MRA or CTA are other alternatives. With multiple TIA's, an angiographic study
should be done. Since the NASCET (North American Symptomatic Carotid Endarterectomy Trial)
study
![]() has proved the efficacy of carotid endarterectomy for stenoses ≥70%, accurate assessment
of the carotid artery is of paramount importance. MRA and CTA are entirely adequate for evaluating
the carotid bifurcations and the circle of Willis. Conventional catheter angiography is generally
reserved for cases where some intervention is being planned, such as thrombolysis, clot removal, or
angioplasty. Conventional angiography is still required in cases of suspected cerebral arteritis where
the more peripheral arteries need to be examined.
has proved the efficacy of carotid endarterectomy for stenoses ≥70%, accurate assessment
of the carotid artery is of paramount importance. MRA and CTA are entirely adequate for evaluating
the carotid bifurcations and the circle of Willis. Conventional catheter angiography is generally
reserved for cases where some intervention is being planned, such as thrombolysis, clot removal, or
angioplasty. Conventional angiography is still required in cases of suspected cerebral arteritis where
the more peripheral arteries need to be examined.
MR Angiography
The two primary methods for MRA are time- of-flight (TOF) and phase contrast (PC). Both use GRE pulse sequences and either 2D or 3D volume acquisitions. TOF makes use of the flow enhancement phenomenon to produce the vascular contrast. PC employs bipolar gradient pulses to tag moving protons with phase shifts. Both produce the MR angiogram by subjecting the data sets to a maximum intensity projection (MIP) ray tracing algorithm to display the vessels from multiple angled views around an axis.
Imaging the neck vessels takes about 10 minutes and can be done in conjunction with a spin-echo scan of the brain. 2D TOF is very good for imaging this area. Overall, accuracy is quite good for estimating stenoses, although the method tends to overestimate the degree and length of stenosis. Very slow flow distal to a critical stenosis is difficult to detect and may be confused with a complete occlusion.
Gadolinium-enhanced MRA can be obtained with a large field of view to visualize the carotid and
vertebral arteries from the aortic arch to the skull base. A 20 cc bolus injection of gadolinium is
followed by a rapid 3D acquisition during passage of the contrast through the arch and neck arteries.
The high contrast-to-noise images are very good for screening for cerebrovascular disease. Tight
stenoses, slow flow, and areas of flow stasis are more accurately assessed with this technique.
![]()
Another application of MRA is in cases of suspected dissection. Relatively minor trauma is sufficient to cause a dissection, or it can be spontaneous. The MRA may demonstrate complete occlusion or only narrowing of the arterial lumen. Spin-echo images should also be obtained because they are very sensitive for detecting the intramural hemorrhage. The typical appearance of an crescent-shaped hyperintensity with an eccentrically placed flow void may be more convincing for a dissection than the MRA. The MRA is very useful for following a dissection to look for recanalization of a complete occlusion or resolution of the vascular compromise caused by the intramural thrombus.
MRA can also evaluate the major intracranial arteries about the circle of Willis. The resolution is lower, and flow artifacts limit accurate assessment of arteriosclerotic disease. Nevertheless, MRA is a very acceptable method for imaging the vertebrobasilar system, which is inaccessible to ultrasound and has no effective surgical therapy. The phase images of 2D PC MRA can be used to determine direction of collateral flow about the circle of Willis.
TOF are PC MRA are very effective in evaluating intracranial veno-occlusive disease, such as superior sagittal sinus thrombosis. PC methods with lower velocity encoding (VENC) factors (15-20 cm/sec) work well for this disease and avoid any possible confusion with thrombus. Thin slab 2D PC can image the full extent of the sagittal sinus in only a few minutes.
CT Angiography
CT angiography is an alternative to MRA. Following a bolus injection of 80-100 ml of iodinated contrast at 3-4 ml/min, a helical acquisition is performed from the lower neck up to the circle of Willis. Images are reconstructed with a 1.25 mm slice thickness at 0.6-1.0 mm increments. In addition to these source images, sagittal reformatted images are obtained to profile the carotid bifurcations. 3D rendered images are also helpful, but they require a tedious interactive approach to carve off the calcified plaques and bone at the skull base.
In general, contrast MRA is a more robust technique for evaluating the carotid arteries. The MIP reconstructions are automatically done by the imaging software. No additional physician time is required for off-line image processing. The entire vascular tree from the aortic arch to the circle of Willis is imaged with a single data acquisition. CTA gives a very good view of the carotid bifurcation and the arteries above the skull base.
REFERENCES