Deep White Matter Ischemia
Pathophysiology
As the brain ages, structural, chemical, and metabolic changes occur that are reflected on
the MR images. Most important for MR interpretation are the changes related to deep white
matter ischemia. The deep white matter of the cerebral hemispheres receives its blood supply
from long, small-caliber arteries and arterioles that penetrate the cerebral cortex and traverse
the superficial white matter fiber tracts. The white matter does not have as generous a blood
supply as the gray matter and is more susceptible to ischemia. As the nutrient arteries become
narrowed by arteriosclerosis and lipohyaline deposits within the vessel walls, the white matter
becomes ischemic on a chronic basis. Pathologically, one of the first changes in the aging
brain is an increase in the perivascular interstitial fluid, predominantly at the arteriolar level
of the vascular tree. With continued progressive ischemia of the white matter, additional
histologic changes are observed, including atrophy of axons and myelin and tortuous,
sclerotic, and thickened vessels. Maintenance of the myelin becomes deficient, resulting in
"myelin pallor" on microscopic sections. Mild gliosis and increased interstitial fluid
accompany the changes in the myelin. Necrosis is not seen until severe ischemia leads to
frank infarction of brain tissue.
![]()
As mentioned previously, the observed changes are secondary to chronic ischemia of the deep white matter and are found more often in patients with ischemic cerebrovascular disease, hypertension, and aging. A combination of arteriolar disease and episodic brain hypoperfusion probably leads to the histopathologic changes. Hypoperfusion can be caused by episodes of hypotension, hypoxia secondary to cardiac or carotid artery disease, hypertension, and/or aging.
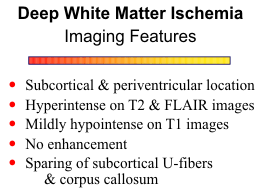
The most common locations for the hyperintensities are the subcortical and periventricular white matter, optic radiations, basal ganglia and brain stem, in decreasing order of frequency. The lesions are hyperintense on T2, proton density, and FLAIR images and have well defined but irregular margins. They are mildly hypointense on T1 weighted images, but the contrast between the lesions and the normal brain is much less than that on T2 weighted images. The white matter changes do not exhibit restriction diffusion, are not associated with breakdown of the blood brain barrier and do not enhance. The process tends to be multifocal, but as the lesions enlarge, a more confluent pattern may develop.
Another pattern of white matter abnormality associated with deep white matter ischemia
is a more or less continuous band of abnormal signal bordering the lateral ventricles.
Normally, interstitial fluid does not accumulate around the ventricles except for small "CSF
caps" around the frontal and occipital horns. White matter ischemia results in mild interstitial
edema that exceeds the capacity of the ependymal transport system. The chronic
accumulation of water around the ventricles adversely affects myelin maintenance and over
time induces partial but irreversible loss of myelin that is reflected as myelin pallor of the
periventricular white matter on pathologic examination. On T2-weighted MR images this
white matter abnormality appears as a hyperintense band of variable thickness located along
the dorsolateral angles of the ventricles, closely apposed to the ependymal surface. A slightly
heterogeneous texture and irregular outer margins distinguish it from the phenomenon of
transependymal CSF flow or "periventricular halo" of obstructive hydrocephalus (see
"Differential Diagnosis" further on). On T1-
weighted images the periventricular lesions
are lower signal than the subcortical white
matter lesions, probably due to increased
water content.
![]()
{To return to cases, use the "Back " button on the Toolbar}