FUNDAMENTALS OF MR SPECTROSCOPY
John R. Hesselink, MD, FACR
MR spectroscopy provides a measure of brain chemistry. The most common nuclei that are used are 1H (proton), 23Na (sodium), 31P (phosphorus). Proton spectroscopy is easier to perform and provides much higher signal-to-noise than either sodium or phosphorus. Proton MRS can be performed within 10-15 minutes and can be added on to conventional MR imaging protocols. It can be used to serially monitor biochemical changes in tumors, stroke, epilepsy, metabolic disorders, infections, and neurodegenerative diseases. The MR spectra do not come labeled with diagnoses. They require interpretation and should always be correlated with the MR images before making a final diagnosis.
BASIC PHYSICAL PRINCIPLES
The
resonant frequencies of nuclei are at the lower end of the electromagnetic
spectrum between FM radio and radar. The
resonant frequencies of protons range between about 10 MHz at 0.3 T to about
300 MHz on a 7 T magnet. The advantages
of higher field strength are higher signal-to-noise and better separation of
the metabolite peaks. In a proton
spectrum at 1.5 T, the metabolites are spread out between 63,000,000 and
64,000,000 Hertz. Rather than use these
large numbers, some very smart person decided to express the resonant
frequencies in parts per million (ppm), and he/she positioned NAA at 2.0 ppm
and let the other metabolites fall into their proper positions on the spectral
line. Then, for unknown reasons, he/she
reversed the ppm scale so that it reads from right to left. 
For MR imaging, the total signal from all the protons in each voxel is used to make the image. If all the signal were used for MRS, the fat and water peaks would be huge and scaling would make the other metabolite peaks invisible. Since we aren't interested in fat and water anyway, the fat and water are eliminated. Fat is avoided by placing the voxel for MRS within the brain, away from the fat in bone marrow and scalp. Water suppression is accomplished with either a CHESS (CHEmical-Shift Selective ) or IR (Inversion Recovery) technique. These suppression techniques are used with a STEAM or PRESS pulse sequence acquisition. A Fourier transform is then applied to the data to separate the signal into individual frequencies. Protons in different molecules resonate at slightly different frequencies because the local electron cloud affects the magnetic field experienced by the proton.
The STEAM (STimulated Echo Acquisition Mode) pulse sequence uses a 90o refocusing pulse to collect the signal like a gradient echo. STEAM can achieve shorter echo times but at the expense of less signal-to-noise. The PRESS (Point REsolved SpectroScopy) sequence refocuses the spins with a 180o rf pulse like a spin echo. Two other acronyms require definition. CSI (Chemical Shift Imaging) refers to multi-voxel MRS. SI (Spectroscopic Imaging) displays the data as an image with the signal intensity representing the concentration of a particular metabolite.
As in MR imaging, the echo time affects the information obtained with MRS. With a short TE of 30 msec, metabolites with both short and long T2 relaxation times are observed. With a long TE of 270 msec, only metabolites with a long T2 are seen, producing a spectrum with primarily NAA, creatine, and choline. One other helpful TE is 144 msec because it inverts lactate at 1.3 ppm.
As a general rule, the single voxel, short TE technique
is used to make the initial diagnosis, because the signal-to-noise is high and
all metabolites are represented.
Multi-voxel, long TE techniques are used to further characterize
different regions of a mass and to assess brain parenchyma around or adjacent
to the mass. Multi-voxel, long TE
techniques are also used to assess response to therapy and to search for tumor
recurrence.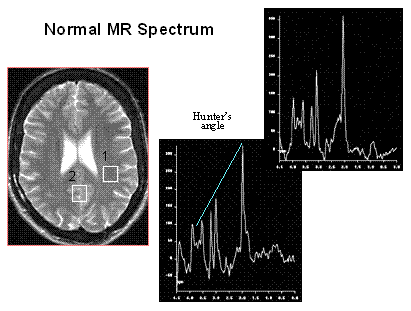
The brain metabolites that are commonly seen on the MR spectrum are listed on the right. Each metabolite appears at a specific ppm, and each one reflects specific cellular and biochemical processes. NAA is a neuronal marker and decreases with any disease that adversely affects neuronal integ-rity. Creatine provides a measure of energy stores. Choline is a measure of increased cellular turnover and is elevated in tumors and inflammatory processes. The observable MR metabolites provide powerful information, but unfortunately, many notable metabolites are not represented in brain MR spectra. DNA, RNA, most proteins, enzymes, and phospholipids are missing. Some key neurotransmitters, such as acetylcholine, dopamine, and serotonin, are absent. Either their concentrations are too low, or the molecules are invisible to MRS.
Normal MR spectra obtained from gray matter and white matter are shown on the right. The predominant metabolites, displayed from right to left, are NAA, creatine, choline, and myo-inositol. The primary difference between the two spectra is that gray matter has more creatine. Hunter's angle is the line formed by the metabolites on the white matter spectrum. The common way to analyze clinical spectra is to look at metabolite ratios, namely NAA/Cr, NAA/Cho, and Cho/Cr. Normal and abnormal values are shown in the chart to the right. By including a known reference solution when acquiring the MR spectral data, absolute concentrations of metabolites can be calculated.
CLINICAL
APPLICATIONS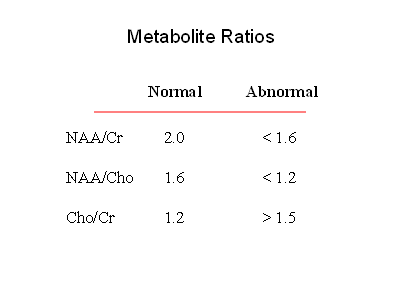
Brain Tumors
MRS can be used to determine the degree of malignancy. As a general rule, as malignancy increases, NAA and creatine decrease, and choline, lactate, and lipids increase. NAA decreases as tumor growth displaces or destroys neurons. Very malig-nant tumors have high metabolic activity and deplete the energy stores, resulting in reduced creatine. Very hypercellular tumors with rapid growth elevate the choline levels. Lipids are found in necrotic portions of tumors, and lactate appears when tumors outgrow their blood supply and start utilizing anaerobic glycolysis. To get an accurate assessment of the tumor chemistry, the spectroscopic voxel should be placed over an enhancing region of the tumor, avoiding areas of necrosis, hemorrhage, calcification, or cysts.
Multi-voxel spectroscopy is best to detect infiltration of malignant cells beyond the enhancing margins of tumors. Particularly in the case of cerebral glioma, elevated choline levels are frequently detected in edematous regions of the brain outside the enhancing mass. Finally, MRS can direct the surgeon to the most metabolically active part of the tumor for biopsy to obtain accurate grading of the malignancy.
A common clinical problem is distinguishing tumor recurrence from radiation effects several months following surgery and radiation therapy. Elevated choline is a marker for recurrent tumor. Radiation change generally exhibits low NAA, creatine, and choline on spectroscopy. If radiation necrosis is present, the spectrum may reveal elevated lipids and lactate.
MRS cannot always distinguish primary and secondary tumors of the brain from one another. As mentioned above, one key feature of gliomas is elevated choline beyond the margin of enhancement due to infiltration of tumor into the adjacent brain tissue. Most non-glial tumors have little or no NAA. Elevated alanine at 1.48 ppm is a signature of meningiomas. They also have no NAA, very low creatine, and elevated glutamates.
Cerebral Ischemia and Infarction
When the brain becomes ischemic, it switches to anaerobic glycolysis and lactate accumulates. Markedly elevated lactate is the key spectroscopic feature of cerebral hypoxia and ischemia. Choline is elevated, and NAA and creatine are reduced. If cerebral infarction ensues, lipids increase.
Trauma
MR spectroscopy is not routinely used in the acute setting of head injuries. CT and MR imaging demonstrate the fractures and intracranial hemorrhage that require emergent surgical intervention. On the other hand, when the patient has stabilized, MRS is helpful to assess the degree of neuronal injury and predict patient outcomes. Especially in the case of diffuse axonal injury, imaging often underestimates the degree of brain damage. Clinical outcome correlates inversely with the NAA/Cr ratio. The presence of any lactate or lipid indicates a worse prognosis.
Infectious Diseases
As in the case of non-glial tumors, brain abscesses destroy or displace brain tissue, so NAA is not present. The voxel should include the abscess cavity to detect the breakdown products of these lesions. Lactate, cytosolic acid, alanine, and acetate are characteristic metabolites in bacterial abscesses. Toxoplasmosis and tuberculomas show prominent peaks from lactate and lipids.
Clinical investigators of HIV infection and AIDS have been very interested in the potential of MRS for measuring the effects of HIV infection on the brain and neuro-cognitive function. Unfortunately, MRS has not proven very sensitive for detecting HIV encephalitis in the early stages of infection. On the other hand when patients start developing neurocognitive deficits and AIDS dementia complex, the MR spectra become positive, namely with elevated choline and reduced NAA. Choline is the best marker for the white matter abnormalities, and the extent of NAA depletion correlates directly with the degree of dementia. MRS is also very helpful in following patients and assessing the effects of anti-viral therapies.
There is also considerable interest in using MRS to distinguish the common focal brain lesions in AIDS patients. The most helpful marker is choline, which is elevated in lymphoma, but low or absent in toxoplasmosis, tuberuloma, and cryptococcoma. Toxoplasmosis is characterized by markedly increased lactate and lipids and depletion of normal brain metabolites. Tuberculoma and cryptococcoma are similar but with relatively little lactate. The spectrum for PML may be similar to lymphoma, but the imaging features are distinctly different and PML may have elevated myo-inositol.
Pediatric Metabolic Disorders
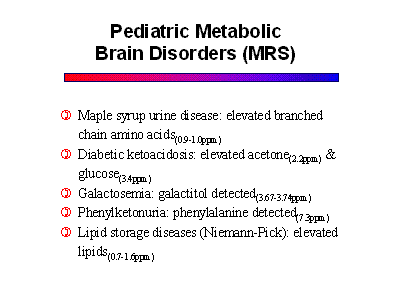
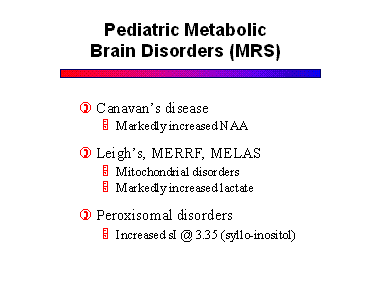
MRS has a very important role in diagnosing and monitoring patients with metabolic disorders.This group includes a long list of diseases that affect the gray and white matter to varying degrees. The names and terminologies of these disorders are confusing because they were derived from the pathologic literature before their metabolic defects were discovered. As the specific biochemical and enzyme defects are being elucidated, these diseases are being classified more appropriately. The list of disorders is long and beyond the scope of this syllabus. Some of the more important diseases are listed below, along with their specific metabolic markers on MR spectra.
Since most metabolic disorders present in infancy, it is important to understand the normal pediatric MR spectrum. Compared to the adult, newborns have much less NAA, and increased choline and myo-inositol. Progression to the adult pattern follows myelination.
Hepatic Encephalopathy
The spectrum of hepatic encephalopathy is characterized by markedly reduced myo-inositol. Choline is also reduced, and glutamine is increased. Liver failure results in excess ammonia in the blood. Ammonia is a neurotoxin and causes increased conversion of glutamate to glutamine. Similar metabolic changes are seen in Reye's syndrome, an acute form of liver failure in infants. The metabolic changes of hepatic encephalopathy increase after a TIPS shunt procedure, and they revert back to normal after successful liver transplantation.
Alzheimer's Disease
Although MR spectroscopy is not highly sensitive for detecting early Alzheimer's disease, as the disease progresses, the spectrum becomes abnormal. Specifically, with advancing disease the NAA is reduced and myo-inositol becomes elevated. Since MRS is totally non-invasive and easily obtained, myo-inositol may become an important marker for assessing new therapies for this devastating disorder.
Myo-inositol is also increased in Down's syndrome, a dementia that presents in childhood and is pathogenetically similar to Alzheimer's disease. On the other hand, myo-inositol is not elevated in other adult dementia, so it is a helpful marker to distinguish Alzheimer's disease from the other causes of dementia.
REFERENCES
1.
Ross BD, Colletti P, Lin A: MR spectroscopy of the brain:
Neurospectroscopy. in Edelman,
Hesselink, Zlatkin & Crues, eds., Clinical Magnetic Resonance Imaging, 3rd
edition, Saunders-Elsevier, Philadelphia, 2006, pp 1840-1910.
- Neuroimaging Clinics,
Volume 3, Number 4, November, 1998.
- Brandao, L, Domigues R.
MR Spectroscopy of the Brain.
Lippincott Williams & Wilkins, 2003.
- Howe FA, Barton SJ, Cudlip SA, et al. Metabolic profiles of human brain tumors
using quantitative in vivo 1H magnetic resonance spectroscopy. Magnetic
Resonance in Medicine 49:223-32, 2003.
- Poptani H, Gupta RK,
Roy R, et al. Characterization of
intracranial mass lesions with in vivo proton MR spectroscopy. AJNR 16:1593-1603, 1995.
- Nelson SJ, McKnight TR,
Henry RG. Characterization of
untreated gliomas by magnetic resonance spectroscopic imaging. Neuroimag Clin 12:599-613, 2002.
- Law M, Cha S, Knopp EA,
et al. High-grade gliomas and solitary metastases: differentiation by
using perfusion and proton spectroscopic MR imaging. Radiology 222:715-721,
2002.
- Graves EE, Nelson SJ, Vigneron DB, et al. Serial proton MR
spectroscopic imaging of recurrent malignant gliomas after gamma knife
radiosurgery. AJNR 22:613-624, 2001.
- Nicoli F, Lefur Y,
Denis B, Ranjeva JP, et al. Metabolic counterpart
of decreased apparent diffusion coefficient during hyperacute ischemic
stroke: a brain proton magnetic resonance spectroscopic imaging study. Stroke
34:82-7, 2003.
- Brenner T, Freier MC,
Holshouser BA, Burley T, Ashwal S. Predicting neuropsychologic outcome
after traumatic brain injury in children. Pediatr Neurol 28:104-14, 2003.
- Chang L, Ernst T. MR spectroscopy and diffusion-weighted
MR imaging in focal brain lesions in AIDS.
Neuroimag Clin 7: 409-426, 1997.
- Lin DD, Crawford TO,
Barker PB. Proton MR spectroscopy in the
diagnostic evaluation of suspected mitochondrial disease. AJNR 24:33-41,
2003.
- Barker PB, Lee RR,
McArthur JC. AIDS dementia complex:
Evaluation with proton MR spectroscopic imaging. Radiology 195:58-64, 1995.